Adsorption Isotherm, Kinetic and Thermodynamic Studies for Removal of Fluoride Ions from Drinking Water Using Modified Natural Pumice
Main Article Content
Abstract
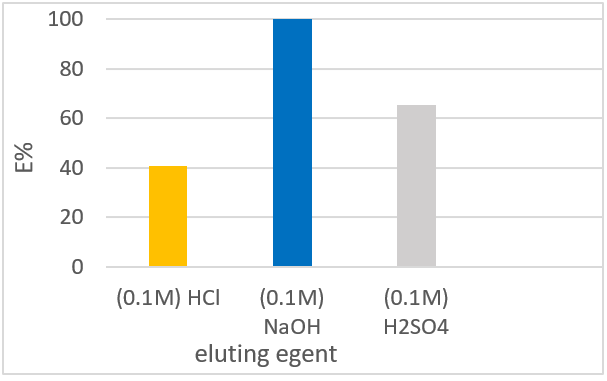
This study aimed to modify pumice using aluminum chloride as an adsorbent for defluoridation. Batch and column experiments were carried out to evaluate the effects of pH, initial fluoride concentration, contact time, dose, and temperatur. The modified pumice adsorbent showed good fluoride removal in both batch and column studies. Fluoride up-take was observed to be effective >7%, at pH 6.5-7.5, and adsorption equilibrium time was observed at 60 min. the adsorption results were modelled using kinetic and isotherm models. Experimental data indicated that fluoride adsorption follows the second pseudo-order kinetics model (R2 > 0.99) and fits well with both the Freundlich and Langmuir isotherm models, which showed favorability for the adsorption of fluoride with a maximum capacity of 0.083 mg/g. The negative values of ∆Ho and ∆Go indicate that the adsorption process was feasible, exothermic, and spontaneous. The column performance showed the best fluoride uptake efficiency, with a breakthrough capacity of 0.48 mg/g. The %R was approximately %100 using0.1M NaOH. Fluoride levels in real wells water decreased dramatically (e.g. 4.1-0.88, 7.1-1.54, and 6.2-1.46 mg/L). The results revealed that modified pumice has the potential for fluoride removal from drinking water.
Downloads
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.